
Baking Soda
also known as Sodium bicarbonate
What is Baking Soda?
Baking soda (NaHCO3) is a type of salt used in baking for leavening dough. It is an alkaline compound that is naturally found in crystalline form, but is used in powdered form for cooking. This substance is often used to help the batter for cakes, muffins, pastries, and breads to rise.
- When combined with an acid, usually in the form of vinegar, lemon juice, or yogurt, it produces carbon dioxide, which gets trapped in the batter, making it rise.
- Also, baking soda is an integral component of baking powder.
Some of the most popular brands are:
- Arm & Hammer
- Pure
- Duda Energy
- ENER-G
- Milliard
- Clabber Girl
Origin of baking soda
For most of human history, the main leavening agent used was yeast. Potassium carbonate began to be used somewhere in the late 1700s, but the process was laborious. Baking soda was first invented by Nicolas Leblanc in the 1790s. The invention of the Solvay process in the 1860s ensured mass production of sodium bicarbonate. Baking powder, derived from this product, was invented in 1846 by Alfred Bird, a British chemist. His wife was allergic to yeast, and Bird tried to help her by inventing an alternative which would offer similar results. He combined sodium bicarbonate and cream of tartar. It quickly became popular because of its ease of use.
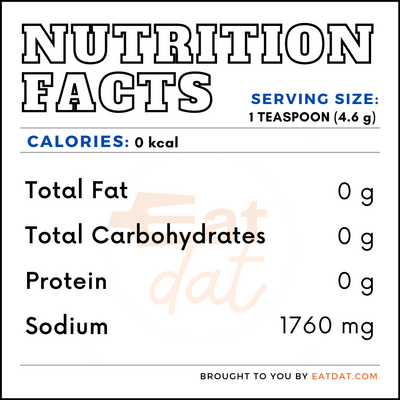
Nutrition
One teaspoon (4.6 g) of baking soda contains:
Baking soda is often used as a cure for gastric problems. In addition, the alkalinity of sodium bicarbonate may help in preventing cancer cells from spreading. However, regular use may lead to metabolic alkalosis, hypokalemia, hypochloremia, hypocalcemia, and hypernatremia, all caused by irregular amounts of alkalinity in the body.
These diseases may severely imbalance the body’s chemicals and lead to mood swings, seizures, and other health issues. Additionally, it may affect the respiratory system, cause heart problems, and induce neurological effects. Furthermore, it may disproportionately affect pregnant women who may experience preeclampsia, and patients using diuretics in order to prevent hypokalemia. Also, it is advised that alcoholics refrain from excessive intake of sodium bicarbonate.
Commercial production
Sodium bicarbonate is a white crystalline powder made by combining an acid (carbonic) with a base (sodium hydroxide). Commercially made baking soda comes from soda ash obtained through the Solvay process or through trona mining. First, the soda ash is centrifugally separated into the liquid and the crystals. Then, the latter is dissolved in a bicarbonate solution, which is later filtered. After that, carbon dioxide is introduced to the solution and sodium bicarbonate crystals form. Finally, the crystals undergo filtration and washing to produce this.
Baking soda recipes
This is mostly used to leaven bread and cakes. Also, it can help caramelization and prevent lumping in certain dishes, or even provide a specific color. Here are a few recipes to try:
- Cloud Bread
- Irish Soda Bread
- Puff Puff
- Kek Sarang Semut
- Nimki
- Pichi Pichi
- Martabak Manis
- Waakye
- Cajeta
- Chocolate Cookies
- Irish Soda Scones
- Vanilla Sponge Cake
- Chocolate Cake
FDA regulations
Baking soda falls under the FDA’s category of food that is generally recognized as safe. It is defined as being the substance obtained by treating a sodium carbonate or a sodium carbonate and sodium bicarbonate solution with carbon dioxide. It must meet the specifications of the Food Chemicals Codex.
References
Alice Graves and Kate Qualmann, The Science of Baking Soda, Axial, ACS Publications, American Chemical Society, https://axial.acs.org/2018/08/03/the-science-of-baking-soda/
Ben Panko, The Great Uprising: How a Powder Revolutionized Baking, Smithsonian Magazine, https://www.smithsonianmag.com/science-nature/great-uprising-how-powder-revolutionized-baking-180963772/
Al-Abri, Suad A, and Kent R Olson. “Baking soda can settle the stomach but upset the heart: case files of the Medical Toxicology Fellowship at the University of California, San Francisco.” Journal of medical toxicology : official journal of the American College of Medical Toxicology vol. 9,3 (2013): 255-8. doi:10.1007/s13181-013-0300-4, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3770998/
